Education
Taking Inspiration From Nature To Make Molecules Like Nature Does

When asked to sign their name, comb their hair, or use a pair of scissors, most humans prefer to use one hand over the other. But being left-handed or right-handed—handedness—is a trait found not just in people. Many molecules, especially organic molecules, exhibit handedness as well.
In some fields of chemistry, particularly in the production of medicines, the handedness of molecules is of vital importance. The drug thalidomide became infamous in the mid-20th century because, while its so-called right-handed version relieves morning sickness, the left-handed version causes severe birth defects. (We will explain in a bit exactly what chemical handedness, also known as chirality, means).
Because that handedness can be so important in chemistry, a great deal of effort has been made to control whether chemical synthesis produces left-handed or right-handed molecules. Now, in a paper published in the journal Science, Hosea Nelson (PhD ’13), Caltech professor of chemistry, describes a new technique devised in his lab that allows for the precise selection of molecular handedness in chemical reactions.
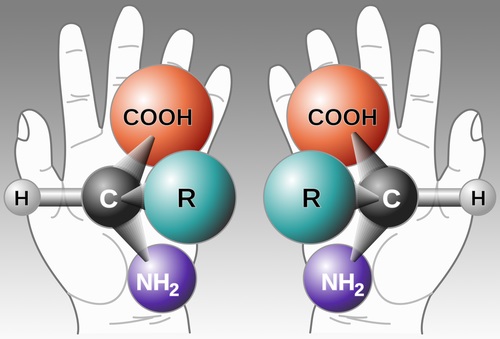
First though, we need to explain what handedness means in molecules. To demonstrate, hold out both of your hands in front of you, palms facing out. The hands are mirror images of each other, right? Now, flip your left hand over. With one palm facing you and one palm facing away, your thumbs both face the same direction and your pinky fingers are on the same side, but your hands are not identical. One hand has fingers that bend away from you, and the other has fingers that bend toward you. No matter which way you turn or rotate either hand, they will always be mirror images of each other rather than identical.
The same is true of some molecules (see the diagram below), a characteristic known in chemistry as chirality (from the Greek word for hand, cheir). For reasons that are not entirely understood, organisms prefer that the molecules they use for specific purposes be one form or the other. Most sugars are right-handed and most amino acids are left-handed, for example.
In the Science paper, Nelson describes a new chemical synthesis technique that is highly selective in making either left-handed or right-handed molecules, depending on what is desired. The technique makes use of a class of molecules called carbocations, which are simply ions in which a carbon atom carries a positive charge.
Carbocations have a long history in chemistry because they are powerfully reactive. Carbocation reactions have been used for the productions of polymers, steroids, and pharmaceutical products, but until now, researchers have not been able to control the reactions in a way that results in the production of only left-handed or right-handed molecules.
However, carbocation reactions are also found throughout the natural world, and they are used to make the proteins, sugars, and many other molecules necessary for every living organism.
“Carbocations are a fundamental building block of all the molecules of life,” Nelson says. “Nature uses them to make molecules of one hand or the other all the time. We haven’t been able to do that.”
But now we can. Using a class of catalysts developed by German chemist Benjamin List (who won the Nobel Prize in chemistry in 2021), Nelson has harnessed carbocation reactions in such a way that they produce only the left-handed or right-handed form of a desired molecule.
These carbocation reactions work by inserting a molecular chunk containing a single carbon atom and hydrogen atom into a precise location in a larger molecule.
On their own, these reactions will produce both forms of the desired molecule. The synthetic catalysts used by Nelson borrow a trick from their natural counterparts, enzymes, to force one form or the other to be made. Both enzymes and the catalysts inspired by them do this by having a cavity in which the reaction takes place. The shape of the cavity dictates which form will be created.
To picture how this works, imagine if you wanted to make a bunch of rubber hands, but you wanted all the hands to be left-handed. One way you could do that would be to get a left-handed glove and then pour liquid rubber into it. After the rubber set, you could pop the rubber hand out of the glove and it would be left handed. A right-handed glove would yield right-handed rubber hands
Nelson says he would like to see the reaction be made more practically applicable in order to move it out of the experimental laboratory setting and into the pharmaceutical chemistry world where he hopes it will eventually be used to synthesize medications more efficiently.
The paper describing the work, titled “Catalytic asymmetric C–H insertion reactions of vinyl carbocations,” appears in the December 9 issue of Science. Co-authors are Caltech graduate chemistry students Sepand K. Nistanaki and Chloe G. Williams; Benjamin Wigman, Jonathan J. Wong, Stasik Popov, and K. N. Houk of UCLA; and Brittany C. Haas, Jacob Werth, formerly of the University of Utah, now with Pfizer, and Matthew S. Sigman of the University of Utah.
Funding for the research was provided by the National Institutes of Health and the National Science Foundation.
Source – Caltech
-

 Auto2 years ago
Auto2 years agoHonda Marine Debuts All-New BF350 Outboard Company’s First V8 Motor Available Commercially, Flagship Model Offers Premium Power and Unparalleled Performance for Extraordinary Boating Experiences
-

 Auto2 years ago
Auto2 years agoNew Features Further Increase Desirability Of Bentayga Range
-

 Technology2 years ago
Technology2 years agoOracle Partners with TELMEX-Triara to Become the Only Hyperscaler with Two Cloud Regions in Mexico
-

 Auto2 years ago
Auto2 years agoHonda and Acura Electric Vehicles Will Have Access to Largest EV Charging Networks in North America Aided by New Agreements with EVgo and Electrify America
-

 Lifestyle2 years ago
Lifestyle2 years ago2023 Nike World Basketball Festival Brings the Best of Basketball Style, Culture and Community